
Compressed gas cylinders combine both a physical hazard, the high pressure, with chemical ones depending on the type of gas. You can find at this link the gas safety training.
For any support, or complementary information, contact us.
In case of an incident or a gas leak
1. Contact the emergency number 115 or +41 21 693 30 00
2. Alarm (if the alarm did not turn on automatically)
3. Evacuate people in danger if possible.
Only the trained and equipped staff can intervene in case of emergency.
If a cylinder gas fallen and looks damaged, do not use it and send it back to the provider.
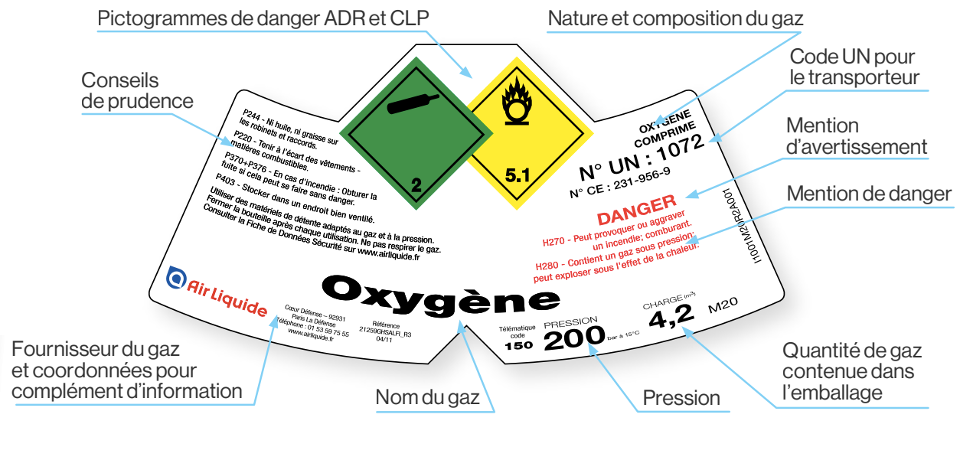
The ADR and CLP symbols, as well as the precautionary advices are first-hand and crucial information sources to identify hazards linked to compressed gases cylinders.
The 4 main gas families are split according to their specific hazards.
Toxic &/or corrosive gases
| Pictograms | 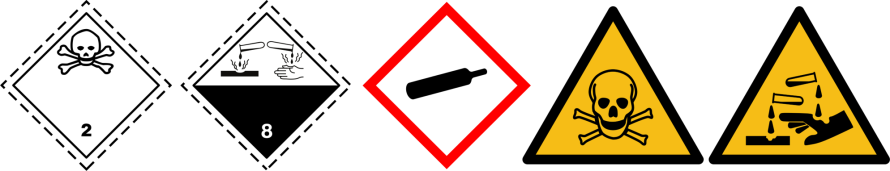 | |
| Gas | Toxic &/or corrosive (ex: H2S, AsH3, PH3, B2H6) | |
| Ogives |  |
Refer to the safety data sheet (SDS) for specific hazards related information of a gas
The filling pressure of gas cylinders can reach up to 300 bars: there is a burst or rupture risk due to the pressure.
With increasing temperature, the pressure of the gas cylinder increases, rising even more the explosion risk. This is well know for liquefied gases such as propane or butane.
A defect on valves or any other safety elements can also cause the incidental release of a large quantity of gas under pressure.
One must not forget:
- All type of gas, even inert, has a grave hazard of asphyxiation.
- A same gaz can hold different chemical properties: hydrogen sulfide (H2S) is toxic, corrosive & flammable.
You can explore the hazards symbols in our page on chemical hazards.
Before the first use
Ensure that the cylinder is equipped with right pressure regulator and certified for the gas. In case of doubt, contact your provider.
Read the safety data sheet for the handling and the safety information concerning the gas you are using. In this document, you will find information on the protective equipment to use when handling this gas. If you have remaining doubts regarding the gas handling, do not hesitate to contact us.
General use
Never re-fill a cylinder by yourself: the remaining gas mixture in a confined space can create unexpected, devastating, and uncontrolled reactions.
Open slowly the valve, without abrupt movement, and entirely. Do not leave a valve partially open. Close the valve when there are long interruptions between the uses and remove the pressure regulator.
Never use grease or oil on the regulator or the valves of the cylinder: these substances can lead to an undesirable and dangerous reaction with the gas contained inside the cylinder.
Never use oxygen as replacement for compressed air.
Depending on the quantity, toxic gases can be monitored by detectors in lab and in cabinets. In case of leak, the detection system stops the gas feed and sends an alarm to the centralized command post – PCC.
According to the internal directive regarding storage of gas cylinders (LEX 1.5.6, PDF, FR), the quantity of gas authorized in a laboratory depends on its type:
Gas toxic or corrosive: maximum 2 cylinders for 0.2 Nm3 total
You can calculate the Nm3 (normal cubic meter) of your cylinders by applying the following formula:
![]()
p = pressure of the cylinder in bar
V = volume in liter
When the cylinder is not used, always put on and tighten the protective cap to protect the main valve.
Cabinets
All gas cylinders must be stored in fireproof cabinets (EI90). The cabinet must be ventilated.
Localisation
- Toxic or corrosive gases are preferably stored inside the laboratory to keep the hazard confined.
- Do not store gas cylinders close to heat sources.
Gas cylinder attachment
A gas cylinder must be permanently secured to a fix point (a wall, a non-movable piece of furniture, etc.):
- at the 2/3 of its height with a chain or a belt for gas cylinder
- one attachment per cylinder
Connectors and regulators
- Adapters of any kind are forbidden.
- Ensure that the cylinder is equipped with the fitting regulator and certified for the specific gas used. if you have any doubt in this concern, please contact your provider.
Never empty entirely a cylinder, always leave a residual pressure of at least 2 bar.
To remember:
- Return empty cylinders to the provider prior to their expiration.
- Empty cylinders and full ones must be stored separately; empty cylinders must be clearly labelled, their valve closed and protected.
Keep the toxic &/or corrosive leaking gas inside the cabinet and call 115.
Depending on the quantity, toxic gases can be monitored by detectors in lab and in cabinets. In case of leak, the detection system stops the gas feed and sends an alarm to the centralized command post – PCC.
If your laboratory uses gases that must be detected, you will have to familiarize yourself with the alarm management procedures developed conjointly by the “Infrastructure and Estate Domain” (DII) and your unit.
Contact us to have more information.

A blinking signal is mounted on top of the laboratory doors (both inside and outside) to alert users of:
- lack of oxygen
- dangerous gas leak

Do not drag, roll, slide a gas cylinder.
To transport safely a gas cylinder, you should only these simple 4 steps:
- Have a three-wheeled cart (“diable”)
- Remove the regulator and put on the protective cap (ogive)
- Fix the cylinder on the cart.
- While transporting: maintain the cylinder with one hand on the protective cap and hold the cart with the other hand.
Flammable gases
| Pictograms | 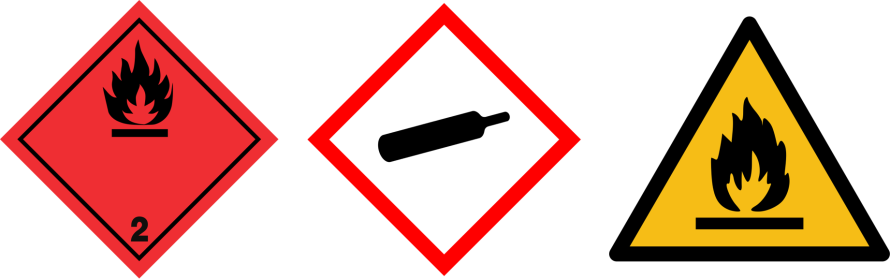 | |||
| Gas | Flammables (ex: H2, CH4, propane) | Acetylene (C2H2) | ||
| Ogives |  |  |
Refer to the safety data sheet (SDS) for specific hazards related information of a gas

The filling pressure of gas cylinders can reach up to 300 bars: there is a burst or rupture risk due to the pressure.
With increasing temperature, the pressure of the gas cylinder increases, rising even more the explosion risk. This is well know for liquefied gases such as propane or butane.
A defect on valves or any other safety elements can also cause the incidental release of a large quantity of gas under pressure.
One must not forget:
- All type of gas, even inert, has a grave hazard of asphyxiation.
- A same gaz can hold different chemical properties: hydrogen sulfide (H2S) is toxic, corrosive & flammable.
You can explore the hazards symbols in our page on chemical hazards.
Before the first use
Ensure that the cylinder is equipped with right pressure regulator and certified for the gas. In case of doubt, contact your provider.
Read the safety data sheet for the handling and the safety information concerning the gas you are using. In this document, you will find information on the protective equipment to use when handling this gas. If you have remaining doubts regarding the gas handling, do not hesitate to contact us.
General use
Never re-fill a cylinder by yourself: the remaining gas mixture in a confined space can create unexpected, devastating, and uncontrolled reactions.
Open slowly the valve, without abrupt movement, and entirely. Do not leave a valve partially open. Close the valve when there are long interruptions between the uses and remove the pressure regulator.
Never use grease or oil on the regulator or the valves of the cylinder: these substances can lead to an undesirable and dangerous reaction with the gas contained inside the cylinder.
Never use oxygen as replacement for compressed air.
Depending on the quantity, toxic gases can be monitored by detectors in lab and in cabinets. In case of leak, the detection system stops the gas feed and sends an alarm to the centralized command post – PCC.
For risk mitigation purposes, for pressure below 10 bar, hydrogen must be produced directly in the laboratory through a hydrogen generator (by water hydrolysis). Ask your safety delegate (CoSEC) or contact us for more information.
Forming gas is a mixture of hydrogen (H2) with nitrogen (N2), or Argon (Ar) or even helium (He). The hydrogen concentration usually vary from 5 to 10 %. This gas is mainly used for the regeneration process of glove-boxes across the campus. For safety reasons (explosive risk related to hydrogen) the concentration of hydrogen in forming gas is limited to a maximum of 5 %. It is not mandatory to detect forming gas.
According to the internal directive regarding storage of gas cylinders (LEX 1.5.6, PDF, FR), the quantity of gas authorized in a laboratory depends on its type:
Gas flammable: maximum 2 cylinders for 0.8 Nm3 total
You can calculate the Nm3 (normal cubic meter) of your cylinders by applying the following formula:
![]()
p = pressure of the cylinder in bar
V = volume in liter
When the cylinder is not used, always put on and tighten the protective cap to protect the main valve.
Cabinets
All gas cylinders must be stored in fireproof cabinets (EI90). The cabinet must be ventilated.
Localisation
- Gas cabinets are normally stored outside of the laboratory.
- Do not store gas cylinders close to heat sources.
Gas cylinder attachment
A gas cylinder must be permanently secured to a fix point (a wall, a non-movable piece of furniture, etc.):
- at the 2/3 of its height with a chain or a belt for gas cylinder
- one attachment per cylinder
Connectors and regulators
- Adapters of any kind are forbidden.
- Ensure that the cylinder is equipped with the fitting regulator and certified for the specific gas used. if you have any doubt in this concern, please contact your provider.
Never empty entirely a cylinder, always leave a residual pressure of at least 2 bar.
To remember:
- Return empty cylinders to the provider prior to their expiration.
- Empty cylinders and full ones must be stored separately; empty cylinders must be clearly labelled, their valve closed and protected.
Keep the flammable leaking gas inside the cabinet and call 115.
Depending on the quantity, toxic gases can be monitored by detectors in lab and in cabinets. In case of leak, the detection system stops the gas feed and sends an alarm to the centralized command post – PCC.
If your laboratory uses gases that must be detected, you will have to familiarize yourself with the alarm management procedures developed conjointly by the “Infrastructure and Estate Domain” (DII) and your unit.
Contact us to have more information.

A blinking signal is mounted on top of the laboratory doors (both inside and outside) to alert users of:
- lack of oxygen
- dangerous gas leak

Do not drag, roll, slide a gas cylinder.
To transport safely a gas cylinder, you should only these simple 4 steps:
- Have a three-wheeled cart (“diable”)
- Remove the regulator and put on the protective cap (ogive)
- Fix the cylinder on the cart.
- While transporting: maintain the cylinder with one hand on the protective cap and hold the cart with the other hand.
Oxidizing gases
| Pictograms | 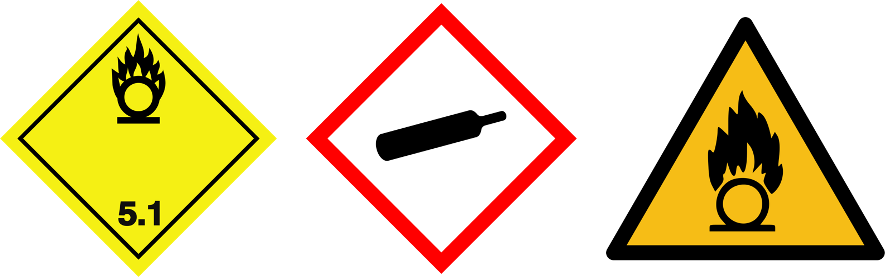 | |||
| Gas | Oxidizer (ex: N2O) | Oxygen (O2) | ||
| Ogives | 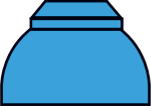 | 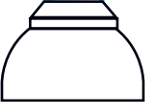 |
Refer to the safety data sheet (SDS) for specific hazards related information of a gas

The filling pressure of gas cylinders can reach up to 300 bars: there is a burst or rupture risk due to the pressure.
With increasing temperature, the pressure of the gas cylinder increases, rising even more the explosion risk. This is well know for liquefied gases such as propane or butane.
A defect on valves or any other safety elements can also cause the incidental release of a large quantity of gas under pressure.
One must not forget:
- All type of gas, even inert, has a grave hazard of asphyxiation.
- A same gaz can hold different chemical properties: hydrogen sulfide (H2S) is toxic, corrosive & flammable.
You can explore the hazards symbols in our page on chemical hazards.
Before the first use
Ensure that the cylinder is equipped with right pressure regulator and certified for the gas. In case of doubt, contact your provider.
Read the safety data sheet for the handling and the safety information concerning the gas you are using. In this document, you will find information on the protective equipment to use when handling this gas. If you have remaining doubts regarding the gas handling, do not hesitate to contact us.
General use
Never re-fill a cylinder by yourself: the remaining gas mixture in a confined space can create unexpected, devastating, and uncontrolled reactions.
Open slowly the valve, without abrupt movement, and entirely. Do not leave a valve partially open. Close the valve when there are long interruptions between the uses and remove the pressure regulator.
Never use grease or oil on the regulator or the valves of the cylinder: these substances can lead to an undesirable and dangerous reaction with the gas contained inside the cylinder.
Never use oxygen as replacement for compressed air.
According to the internal directive regarding storage of gas cylinders (LEX 1.5.6, PDF, FR), the quantity of gas authorized in a laboratory depends on its type:
Oxidizing gas: maximum 2 cylinders for 0.8 Nm3 total
You can calculate the Nm3 (normal cubic meter) of your cylinders by applying the following formula:
![]()
p = pressure of the cylinder in bar
V = volume in liter
When the cylinder is not used, always put on and tighten the protective cap to protect the main valve.
Cabinets
All gas cylinders must be stored in fireproof cabinets (EI90). The cabinet must be ventilated.
Localisation
- Gas cabinets are normally stored outside of the laboratory.
- Do not store gas cylinders close to heat sources.
Gas cylinder attachment
A gas cylinder must be permanently secured to a fix point (a wall, a non-movable piece of furniture, etc.):
- at the 2/3 of its height with a chain or a belt for gas cylinder
- one attachment per cylinder
Connectors and regulators
- Adapters of any kind are forbidden.
- Ensure that the cylinder is equipped with the fitting regulator and certified for the specific gas used. if you have any doubt in this concern, please contact your provider.
Never empty entirely a cylinder, always leave a residual pressure of at least 2 bar.
To remember:
- Return empty cylinders to the provider prior to their expiration.
- Empty cylinders and full ones must be stored separately; empty cylinders must be clearly labelled, their valve closed and protected.
Keep the oxidizing leaking gas in a ventilated space and call 115.
If your laboratory uses gases that must be detected, you will have to familiarize yourself with the alarm management procedures developed conjointly by the “Infrastructure and Estate Domain” (DII) and your unit.
Contact us to have more information.

A blinking signal is mounted on top of the laboratory doors (both inside and outside) to alert users of:
- lack of oxygen
- dangerous gas leak
Do not drag, roll, slide a gas cylinder.
To transport safely a gas cylinder, you should only these simple 4 steps:
- Have a three-wheeled cart (“diable”)
- Remove the regulator and put on the protective cap (ogive)
- Fix the cylinder on the cart.
- While transporting: maintain the cylinder with one hand on the protective cap and hold the cart with the other hand.
Inert gases
| Pictograms | 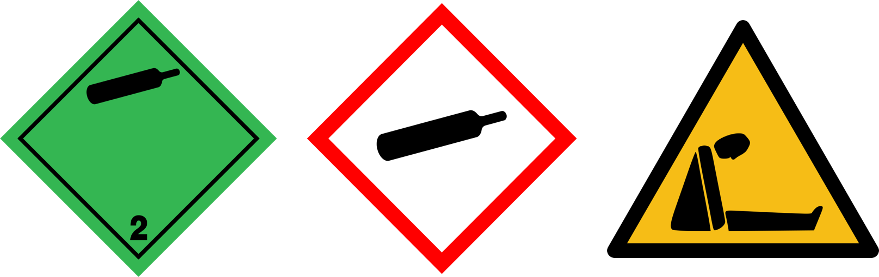 | |||
| Gases | Asphyxiant / inert (ex: Ar) | Nitrogen (N2) | Carbon dioxyde (CO2) | Helium (He) |
| Ogives |  |  |  |  |
Refer to the safety data sheet (SDS) for specific hazards related information of a gas

The filling pressure of gas cylinders can reach up to 300 bars: there is a burst or rupture risk due to the pressure.
With increasing temperature, the pressure of the gas cylinder increases, rising even more the explosion risk. This is well know for liquefied gases such as propane or butane.
A defect on valves or any other safety elements can also cause the incidental release of a large quantity of gas under pressure.
One must not forget:
All type of gas, even inert, has a grave hazard of asphyxiation.
You can explore the hazards symbols in our page on chemical hazards.
Before the first use
Ensure that the cylinder is equipped with right pressure regulator and certified for the gas. In case of doubt, contact your provider.
Read the safety data sheet for the handling and the safety information concerning the gas you are using. In this document, you will find information on the protective equipment to use when handling this gas. If you have remaining doubts regarding the gas handling, do not hesitate to contact us.
General use
Never re-fill a cylinder by yourself: the remaining gas mixture in a confined space can create unexpected, devastating, and uncontrolled reactions.
Open slowly the valve, without abrupt movement, and entirely. Do not leave a valve partially open. Close the valve when there are long interruptions between the uses and remove the pressure regulator.
Never use grease or oil on the regulator or the valves of the cylinder: these substances can lead to an undesirable and dangerous reaction with the gas contained inside the cylinder.
Never use oxygen as replacement for compressed air.
A CO2 detector must be present if the concentration in the laboratory can reach or exceed the threshold value of 5000 ppm (ml/m3).
Contact us to have more information.
Forming gas is a mixture of hydrogen (H2) with nitrogen (N2), or Argon (Ar) or even helium (He). The hydrogen concentration usually vary from 5 to 10 %. This gas is mainly used for the regeneration process of glove-boxes across the campus. For safety reasons (explosive risk related to hydrogen) the concentration of hydrogen in forming gas is limited to a maximum of 5 %. It is not mandatory to detect forming gas.
According to the internal directive regarding storage of gas cylinders (LEX 1.5.6, PDF, FR), the quantity of gas authorized in a laboratory depends on its type:
Inert gases: maximum 4 cylinders for 2 Nm3 total
You can calculate the Nm3 (normal cubic meter) of your cylinders by applying the following formula:
![]()
p = pressure of the cylinder in bar
V = volume in liter
When the cylinder is not used, always put on and tighten the protective cap to protect the main valve.
Cabinets
All gas cylinders must be stored in fireproof cabinets (EI90). The cabinet must be ventilated.
Localisation
- Gas cabinets are normally stored outside of the laboratory.
- Do not store gas cylinders close to heat sources.
Gas cylinder attachment
A gas cylinder must be permanently secured to a fix point (a wall, a non-movable piece of furniture, etc.):
- at the 2/3 of its height with a chain or a belt for gas cylinder
- one attachment per cylinder
Connectors and regulators
- Adapters of any kind are forbidden.
- Ensure that the cylinder is equipped with the fitting regulator and certified for the specific gas used. if you have any doubt in this concern, please contact your provider.
Never empty entirely a cylinder, always leave a residual pressure of at least 2 bar.
To remember:
- Return empty cylinders to the provider prior to their expiration.
- Empty cylinders and full ones must be stored separately; empty cylinders must be clearly labelled, their valve closed and protected.
Store the leaking cylinder in the open air, if the gas is inert or in an artificially ventilated zone otherwise. Label it as leaking and contact the provider.
- CO2 detectors and oxygen lack detectors are installed if necessary.
- A CO2 detector must be present if the concentration in the laboratory can reach or exceed the threshold value of 5000 ppm (ml/m3).
If your laboratory uses gases that must be detected, you will have to familiarize yourself with the alarm management procedures developed conjointly by the “Infrastructure and Estate Domain” (DII) and your unit.
Contact us to have more information.

A blinking signal is mounted on top of the laboratory doors (both inside and outside) to alert users of:
- lack of oxygen
- dangerous gas leak

Do not drag, roll, slide a gas cylinder.
To transport safely a gas cylinder, you should only these simple 4 steps:
- Have a three-wheeled cart (“diable”)
- Remove the regulator and put on the protective cap (ogive)
- Fix the cylinder on the cart.
- While transporting: maintain the cylinder with one hand on the protective cap and hold the cart with the other hand.