Single-cell manipulation and analysis
The cell is one of the fundamental building blocks of life. Their understanding is thus of tremendous importance to understand cellular processes, assess the influence of physical and find new ways to treat diseases related to cellular death. An underlying issue is the heterogeneity of cell populations that can mask important differences between individual cells. High-throughput single-cell analysis can bridge this knowledge gap, breaking down results to the minimal functional unit of organic matter, the cell.
In the Laboratory of Life Science Electronics (CLSE), we develop new microfluidic techniques enabling single-cell manipulation and analysis.
Electrorotation is a technique to investigate the dielectric properties of bioparticles. We have developed a microfluidic device consisting of an array of 39 micro cages to enable high-throughput measurement of the membrane capacitance of a single cell. The membrane capacitance can give insight into the dielectric properties and the integrity of the lipid bilayer of a eukaryotic cell [1]. We have shown that we are able to distinguish between different cell lines using our device [1]. Furthermore, we plan to investigate the impact of amyloidogenic proteins related to the Parkinson’s disease on the membrane using electrorotation to contribute to a better understanding of neurodegenerative diseases.
…

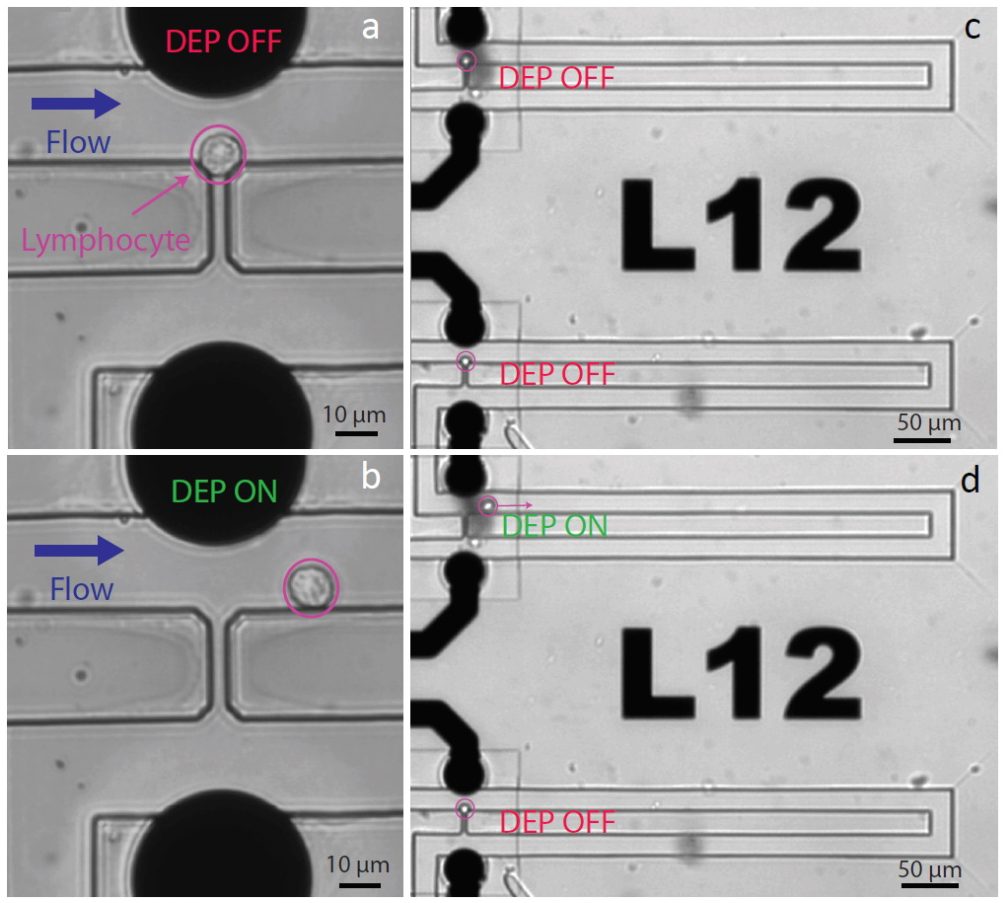
In another project, we enabled the capture of single cells using hydrodynamic traps. The device made it possible to release single cells selectively using dielectrophoresis, recover them and analyze their transcriptome by DropSeq [2]. We investigated the stress induced on cells by our platform through analysis of the molecular phenotype, revealing no impact of DEP application on the transcriptional signature of the cells. In general, this device could be used for controlled monitoring of single-cells that were subjected to different drugs or toxins.