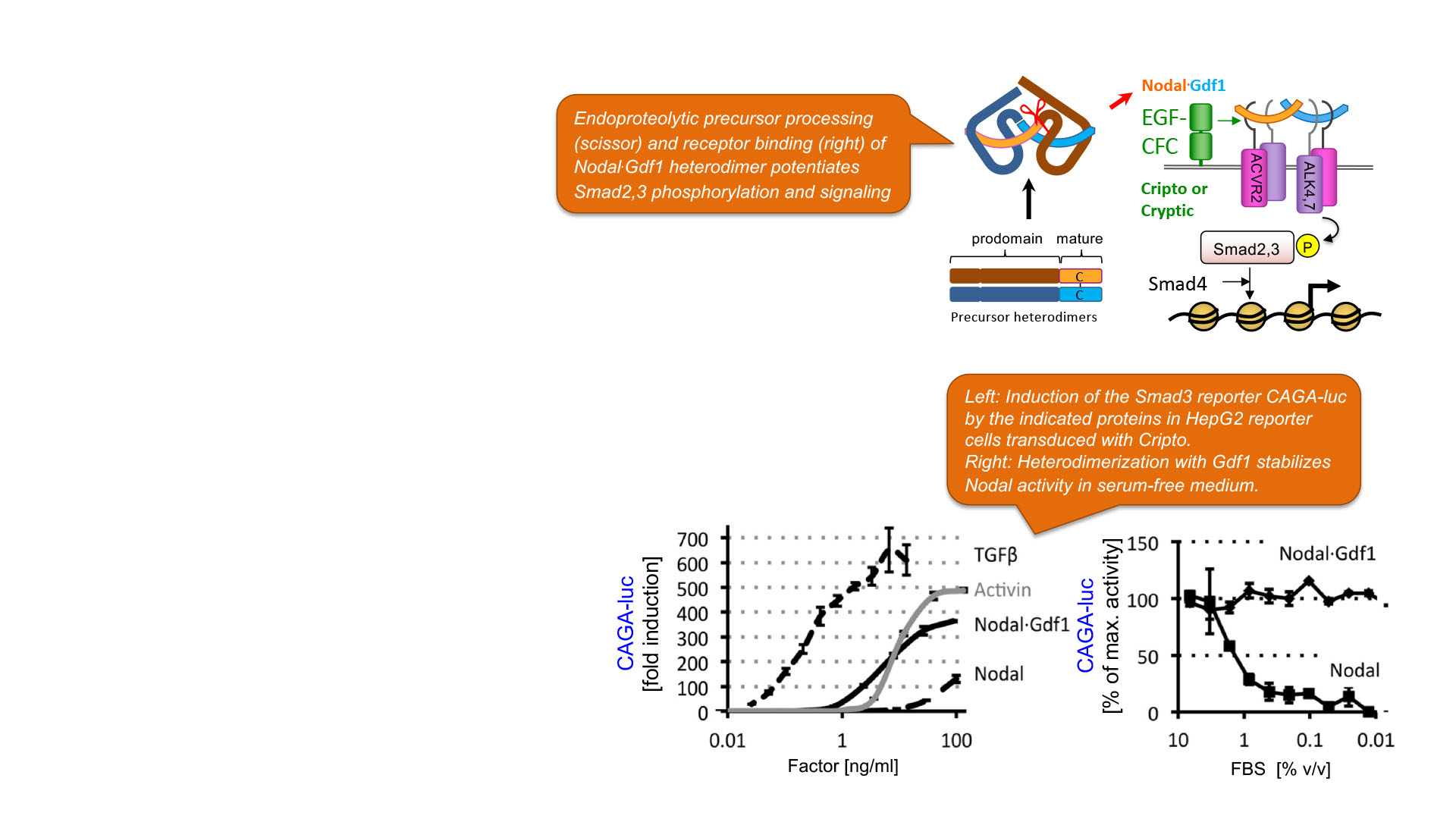
TGFβ-related signals mediated by Nodal and Gdf1(3)
Expansion of pluripotent stem cells and their lineage differentiation in mammals are coordinated by secreted heterodimers of Nodal and Gdf1 (or Gdf3) and their EGF-CFC co-receptors. Human NODAL or GDF1,3 are also sporadically transcribed in certain solid tumours e.g. in prostate, breast and brain. However, since these ligands only signal efficiently when they are co-expressed as heterodimers and with co-receptors, the probability that they are co-opted to promote tumour progression is limited.
Activin-A is encoded by the Inhibin βA gene
Other Activin receptor ligands are more frequently implicated in cancer, including Activin-A. Activin-A is formed by Inhibin βA chains that homodimerize prior to ER exit. In the circulation, mature Activins are sequestered by secreted antagonists. In addition, the bioavailability of Activins is limited by heterodimerization of gonadal Inhibin β with Inhibin α subunits. In Inhibin α knockout mice, excessive release of Activins increases morbidity and mortality by inducing systemic muscle wasting, indicating that neutralization of free Activin is important. Gain of Activin-A signaling can also accelerate or slow tumorigenesis. However, what determines in a given tissue whether Activin-A is oncogenic or tumor suppressive remains unclear.
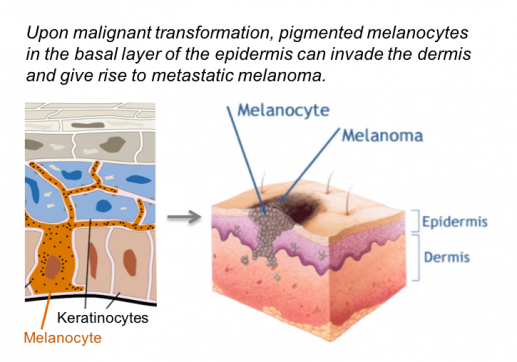
The INHβA gene is frequently upregulated in several tumor types, including melanoma and other skin cancers. To block Activin-A signaling, we expressed truncated type II receptor in xenografts of metastatic human melanoma cells. While suppression of tumor-induced muscle wasting (cachexia) confirmed that the ligand trap was functional, primary tumor growth and lung metastases were unaffected (Donovan et al., 2017).
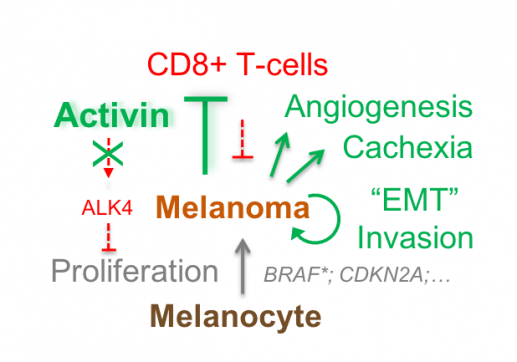
To test whether an oncogenic function of Activin-A was curtailed by the lack of anti-tumor immunity, we used wild-type or immunodeficient Rag-1-/- mice to syngeneically graft mouse melanoma expressing lentiviral INHβA or Nodal or a constitutively active mutant form of their type I receptor ALK4. We found that Activin-A secretion by INHβA expressing melanoma cells reduces cytotoxic T cell infiltration and accelerates tumour growth and experimental lung metastases specifically in wild-type hosts, but not in immunodeficient hosts. In sharp contrast, cell autonomous ALK4 signaling within melanoma cells abolished tumour growth rather than stimulating it.
What determines whether Activin promotes or rather inhibits tumorigenesis?
Our findings indicate that the net effect of secreted Activin-A on melanoma progression likely depends on the balance between cell autonomous (autocrine) and cell non-autonomous (paracrine) signaling. How this balance is regulated and whether anti-tumour immunity can be boosted by inhibiting Activin signaling to the tumour microenvironment remains to be determined.
Publications related to this project
P. Donovan; O. A. Dubey; S. Kallioinen; K. W. Rogers; K. Muehlethaler; P. Müller; D. Rimoldi; D. B. Constam. Paracrine Activin-A signaling promotes melanoma growth and metastasis through immune evasion; Journal of Investigative Dermatology. 2017. DOI : 10.1016/j.jid.2017.07.845.
C. Fuerer; M. C. Nostro; D. B. Constam : Nodal.Gdf1 heterodimers with bound prodomains enable serum-independent Nodal signaling and endoderm differentiation; Journal Biological Chemistry. 2014. DOI : 10.1074/jbc.M114.550301.